-
Sign In
-

-
 Sony Biotechnology
Sony Biotechnology
-
-
 Sony Biotechnology
Sony Biotechnology
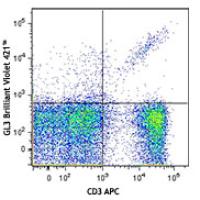
Brilliant Violet 421™ anti-mouse TCR γ/δ
Antibodies Single
Sony
GL3
Flow Cytometry
Hamster IgG
Mouse
C57BL/6J intraepithelial lymphocytes
1190600
$267.00
Description
T cell receptor (TCR) is a heterodimer consisting of an α and a β chain (TCR α/β) or a γ and a δ chain (TCR γ/δ). TCR γ/δ belongs to the immunoglobulin superfamily, which is involved in the recognition of certain bacterial and tumor antigens bound to MHC class I. γ/δ TCR associates with CD3 and is expressed on a T cell subset found in the thymus, the intestinal epithelium, and the peripheral lymphoid tissues and peritoneum. Most γ/δ T cells are CD4-/CD8- although some are CD8+. T cells expressing the γ/δ TCR have been shown to play a role in oral tolerance, tumor-associated tolerance, and autoimmune disease. It has been reported that γ/δ T cells also play a principal role in antigen presentation.
Formulation
Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide and BSA (origin USA).Recommended Usage
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For immunofluorescent staining using the microg size, the suggested use of this reagent is ≤0.25 microg per million cells in 100 microL volume. For immunofluorescent staining using the microL size, the suggested use of this reagent is ≤5 microL per million cells or 5 microL per 100 microL of whole blood. It is recommended that the reagent be titrated for optimal performance for each application.
Brilliant Violet 421™ excites at 405 nm and emits at 421 nm. The standard bandpass filter 450/50 nm is recommended for detection. Brilliant Violet 421™ is a trademark of Sirigen Group Ltd.
This product is subject to proprietary rights of Sirigen Inc. and is made and sold under license from Sirigen Inc. The purchase of this product conveys to the buyer a non-transferable right to use the purchased product for research purposes only. This product may not be resold or incorporated in any manner into another product for resale. Any use for therapeutics or diagnostics is strictly prohibited. This product is covered by U.S. Patent(s), pending patent applications and foreign equivalents.
References
1. Goodman T, et al. 1989. J. Exp. Med. 170:1569. (FC, IP)
2. Cardona AE, et al. 2003. Infect. Immun. 71:2634. (IHC)
3. Kapp JA, et al. 2004. Immunology 111:155. (Deplete)
4. Skelsey ME, et al. 2001. J. Immunol. 166:4327. (Deplete)
5. Ke Y, et al. 1997. J. Immunol. 158:3610. (Deplete)
6. Podd BS, et al. 2006. J. Immunol. 176:6532. (IHC)
7. Kasten KR, et al. 2010. Infect. Immun. 78:4714. (FC) PubMed
8. Stadanlick JE, et al. 2011. J. Immunol. 187:664. PubMed
9. Van Belle AB, et al. 2012. J. Immunol. 188:462. PubMed
10. Blanco R, et al. 2014. Sci Signal. 2:354. PubMed