-
Sign In
-

-
 Sony Biotechnology
Sony Biotechnology
-
-
 Sony Biotechnology
Sony Biotechnology
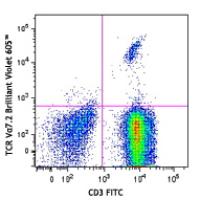
Brilliant Violet 605™ anti-human TCR Vα7.2
Antibodies Single
Sony
3C10
Flow Cytometry
Mouse IgG1, κ
Human
Recombinant TCR
2358595
$226.00
Description
The 3C10 antibody recognizes the Vα7.2 T cell antigen receptor (TCR) α-chain segment which, joined with the Jα33 segment, constitutes an invariant TCR that is a characteristic of the mucosal-associated invariant T cells (MAIT cells). MAIT cells are restricted by a nonpolymorphic class Ib major histocompatibility complex (MHC) molecule, MHC-related molecule 1 (MR1). MAIT cells are present in human blood (1-8% of T cells), mesenteric lymph nodes, liver, and intestinal mucosa. MAIT cells play a role in detecting and fighting off microbial infections.
Formulation
Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide and BSA (origin USA).Recommended Usage
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤5 microL per million cells or 5 microL per 100 microL of whole blood. It is recommended that the reagent be titrated for optimal performance for each application.
Brilliant Violet 605™ excites at 405 nm and emits at 603 nm. The bandpass filter 610/20 nm is recommended for detection, although filter optimization may be required depending on other fluorophores used. Be sure to verify that your cytometer configuration and software setup are appropriate for detecting this channel. Refer to your instrument manual or manufacturer for support. Brilliant Violet 605™ is a trademark of Sirigen Group Ltd.
This product is subject to proprietary rights of Sirigen Inc. and is made and sold under license from Sirigen Inc. The purchase of this product conveys to the buyer a non-transferable right to use the purchased product for research purposes only. This product may not be resold or incorporated in any manner into another product for resale. Any use for therapeutics or diagnostics is strictly prohibited. This product is covered by U.S. Patent(s), pending patent applications and foreign equivalents.
References
1. Martin E, et al. 2009. PLoS Biol. 7:525.
2. Wakao H, et al. 2013. Cell Stem Cell 12:1. PubMed