-
Sign In
-

-
 Sony Biotechnology
Sony Biotechnology
-
-
 Sony Biotechnology
Sony Biotechnology
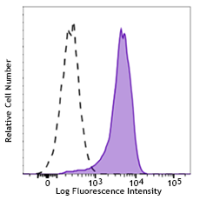
Brilliant Violet 421™ anti-STAT6 Phospho (Tyr641)
Antibodies Single
Sony
A15137E
Intracellular Staining for Flow Cytometry
Mouse IgG1, κ
Human
Human STAT6 peptide phosphorylated at Tyr 641
4030100
$457.00
Description
STAT6 is a member of the signal transducer and activator of transcription (STAT) family, activating gene expression in response to IL-4 and IL-13 stimulation. Upon cytokine stimulation, the receptor is phosphorylated by the associated Janus Kinases (Jak), followed by recruiting cytoplasmic STAT6. The Tyr641 residue of STAT6 is, in turn, phosphorylated by Jak. Phosphorylated STAT6 forms homodimers, translocates to the nucleus, and regulates transcription of target genes. STAT6 plays crucial roles in differentiation of T helper 2 (Th2) cells, class switch of immunoglobulins in B cells, expression of cell surface markers such as MHC class II, and the development of allergic inflammation.
Formulation
Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide and BSA (origin USA)Recommended Usage
Each lot of this antibody is quality control tested by intracellular flow cytometry using our True-Phos™ Perm Buffer in Cell Suspensions Protocol. For flow cytometric staining, the suggested use of this reagent is 5 µL per million cells in 100 µL staining volume or 5 µL per 100 µL of whole blood. It is recommended that the reagent be titrated for optimal performance for each application.
Brilliant Violet 421™ excites at 405 nm and emits at 421 nm. The standard bandpass filter 450/50 nm is recommended for detection. Brilliant Violet 421™ is a trademark of Sirigen Group Ltd.
This product is subject to proprietary rights of Sirigen Inc. and is made and sold under license from Sirigen Inc. The purchase of this product conveys to the buyer a non-transferable right to use the purchased product for research purposes only. This product may not be resold or incorporated in any manner into another product for resale. Any use for therapeutics or diagnostics is strictly prohibited. This product is covered by U.S. Patent(s), pending patent applications and foreign equivalents.
References
- Tsujisaki M, et al. 1991. Clin. Exp. Immunol. 85:3.
- Kanwar JR, et al. 2003. Cancer Gene Ther. 10:468.
- Kohka H, et al. 1998. J. Leukoc. Biol. 64:519.