-
Sign In
-

-
 Sony Biotechnology
Sony Biotechnology
-
-
 Sony Biotechnology
Sony Biotechnology
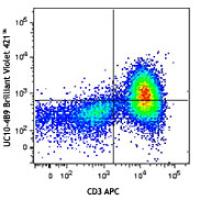
Brilliant Violet 421™ anti-mouse CD152
Antibodies Single
Sony
UC10-4B9
Flow Cytometry
Hamster IgG
Mouse
Mouse CTLA-4-mouse IgG2a fusion protein
1131560
$272.00
Description
CD152, also known as CTLA-4 or Ly-56, is a 33 kD member of the immunoglobulin superfamily. It is expressed on activated T and B lymphocytes. CD152 is similar to CD28 in amino acid sequence, structure, and genomic organization and these two receptors share common B7 family counter-receptors (B7-1, B7-2). Whereas CD28 delivers a costimulatory signal in T cell activation, CTLA-4 negatively regulates cell-mediated immune responses. CD152 is thought to play a role in the induction and maintenance of immunological tolerance as well as the development of protective immunity and thymocyte regulation.
Formulation
Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide and BSA (origin USA).Recommended Usage
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For immunofluorescent staining using the microg size, the suggested use of this reagent is ≤1.0 microg per million cells in 100 microL volume. For immunofluorescent staining using the microL size, the suggested use of this reagent is ≤5 microL per million cells or 5 microL per 100 microL of whole blood. It is recommended that the reagent be titrated for optimal performance for each application.
Brilliant Violet 421™ excites at 405 nm and emits at 421 nm. The standard bandpass filter 450/50 nm is recommended for detection. Brilliant Violet 421™ is a trademark of Sirigen Group Ltd.
This product is subject to proprietary rights of Sirigen Inc. and is made and sold under license from Sirigen Inc. The purchase of this product conveys to the buyer a non-transferable right to use the purchased product for research purposes only. This product may not be resold or incorporated in any manner into another product for resale. Any use for therapeutics or diagnostics is strictly prohibited. This product is covered by U.S. Patent(s), pending patent applications and foreign equivalents.
References
1. Walunas TL, et al. 1994. Immunity 1:405. (Block, IP)
2. Cilio CM, et al. 1998. J. Exp. Med. 188:1239. (Block)
3. Issazadeh S, et al. 1999. J. Immunol. 162:761. (Block)
4. McCoy K, et al. 1997. J. Exp. Med. 186:183. (Block)
5. Hsu HC, et al. 2007. J. Immunol. 178:5357. (ELISA Capture)
6. Sugita S, et al. 2010. Invest. Ophthalmol. Vis. Sci. 51:5783. PubMed