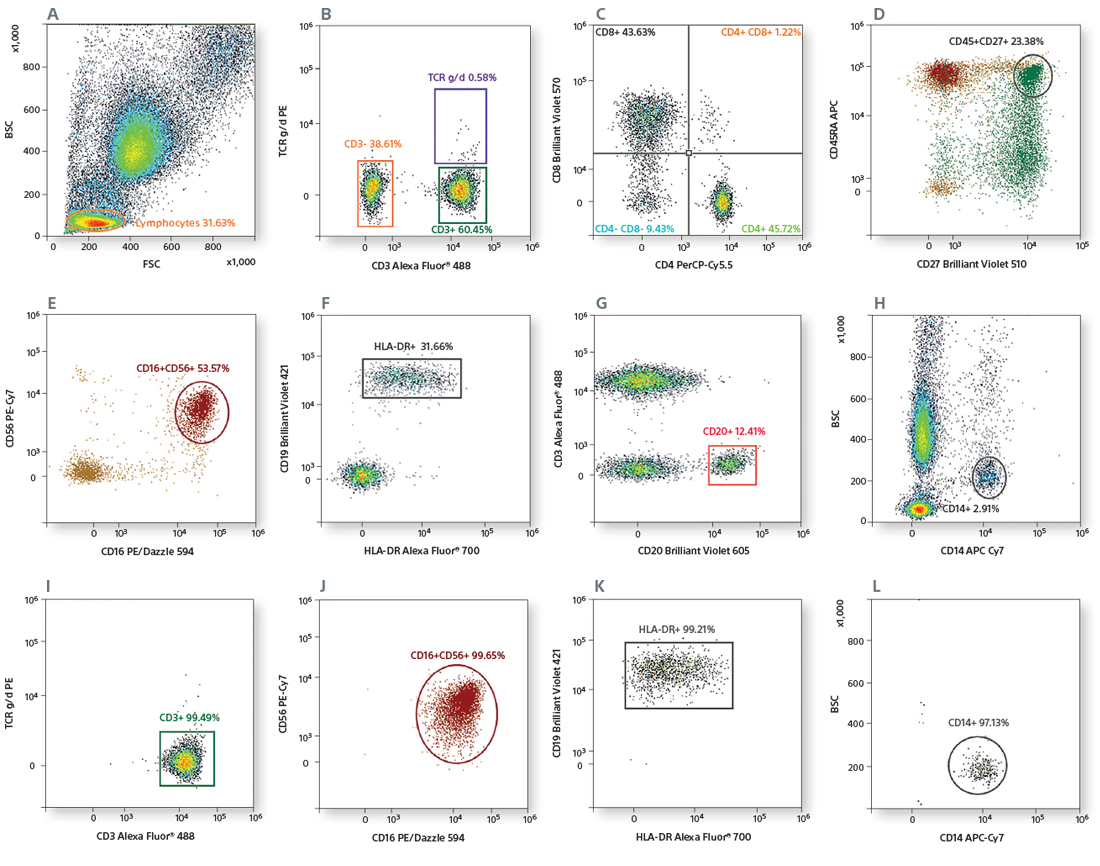
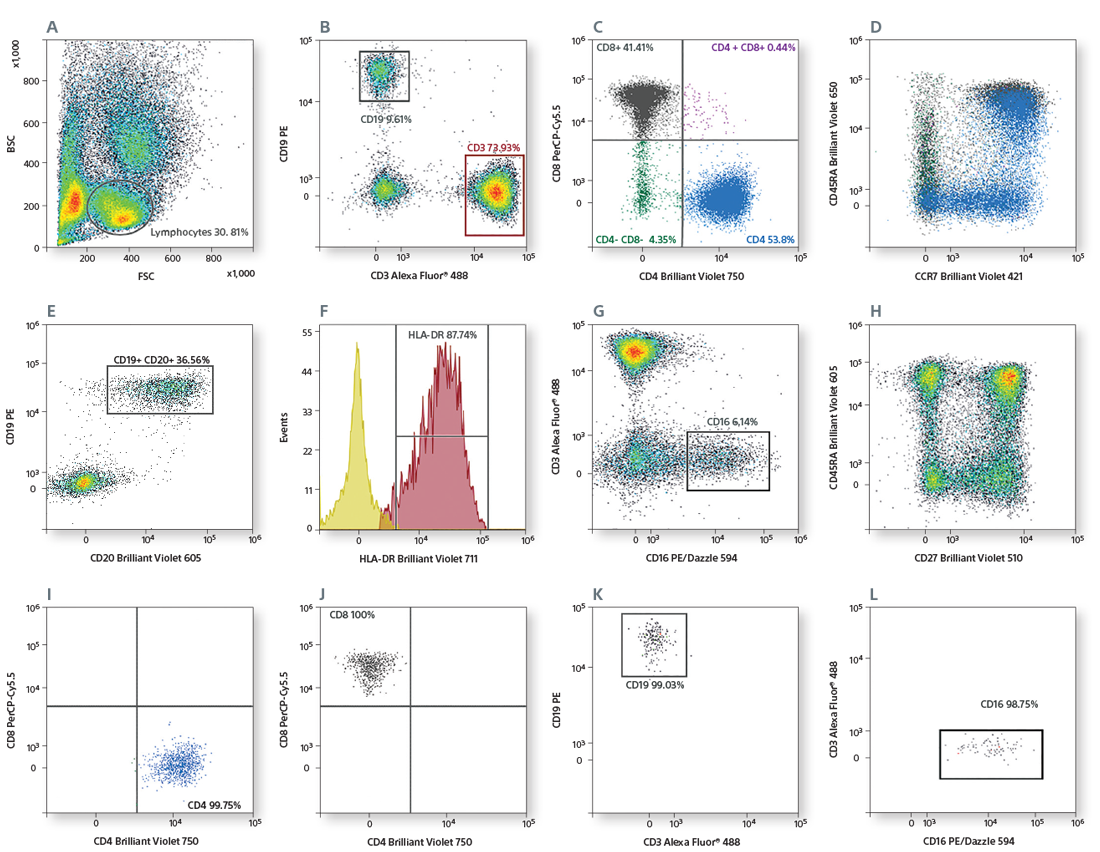
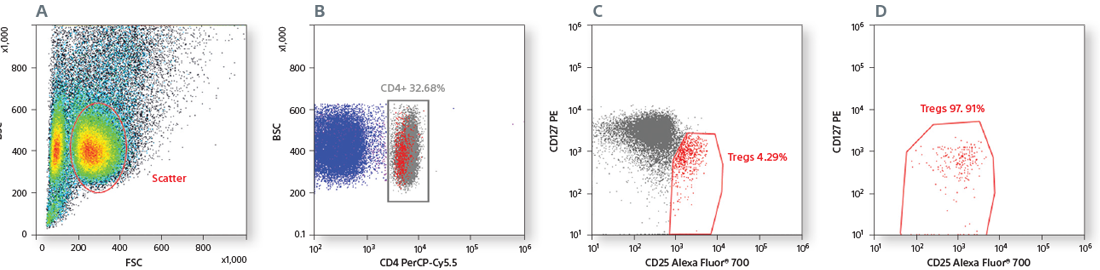
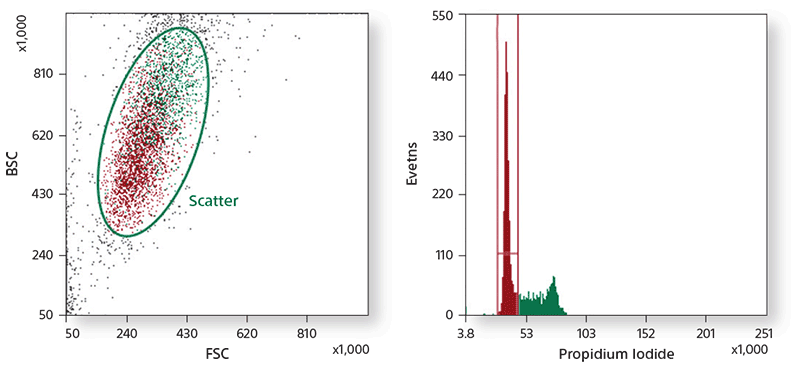
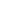Scatter was used for gating lymphocytes (A). The CD3+ population (B) was used to gate CD4+ and CD8+ cells (C). CD4+ T-cell subsets were identified based on CD45RA and CD27 expression (D). CD16+CD56+ NK cells were gated from CD3- cells (E). CD19+CD20+ B cells were gated from CD3- cells (F), and the HLA-DR expression of B cells was analyzed (G). CD14+ monocytes were identified based on scatter (H). CD3+ T cells, CD19+CD20+ B cells, CD16+CD56+ NK cells, and CD14+ monocytes were sorted by 4-way sorting. Post-sort analysis of each sorted population is shown (I–L).